Common blood pressure drugs recalled over fears ingredient ‘increases cancer risk’
and live on Freeview channel 276
Blood pressure drugs commonly used to treat NHS patients have been recalled by the Medicines and Healthcare products Regulatory Agency (MHRA), due to risks of contamination.
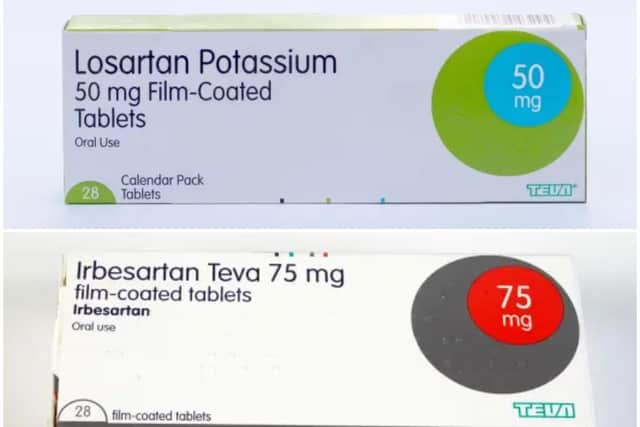
Thirty one batches of products containing Losartan or Irbesartan have been recalled, as fears grow they could be contaminated by a substance which increases the risk of cancer.
Advertisement
Hide AdAdvertisement
Hide AdWe want to hear from you: let us know what you think about this story and be part of the debate in our comments section below

'It’s important that healthcare professionals check their stock'
Despite the risk, the MHRA have advised that even if you currently take a prescription with these drugs in them, that you continue to take your medication.
If you are concerned that your medication may be infected, you should contact your GP practice and discuss this with your doctor.
At present (17 June), there is no reason to believe that a patient has been harmed by the possible contamination and the medical regulatory body are recalling tablets on a precautionary basis.
Advertisement
Hide AdAdvertisement
Hide AdPharmacists and wholesalers have been contacted and for now, this is not a recall at patient-level. The affected batch numbers can be found on the NHS website.
Dr June Raine, MHRA Chief Executive, said: “Patient safety is our watchword.
"We’re recalling batches of certain sartan-containing products as a precautionary measure while we continue our investigation.
"It’s important that healthcare professionals check their stock to quarantine and return these batches.
Advertisement
Hide AdAdvertisement
Hide Ad“If you’ve been taking one of the affected products, speak with your doctor or pharmacist before stopping any treatment – they can address any concerns and can advise you on the best course of action.”
The MHRA continues to work with other regulatory health bodies to manage the ongoing situation and is reviewing other recalls of these types of products, which took place in 2018 and 2019.
Due to the minor risk to patient health from the possible contamination, patients are asked to continue taking their medication as blood pressure must be regulated.
Not doing so could lead to high blood pressure, which could prove problematic and may require further medical attention.
Comment Guidelines
National World encourages reader discussion on our stories. User feedback, insights and back-and-forth exchanges add a rich layer of context to reporting. Please review our Community Guidelines before commenting.